Abstract
Background: trALL is an important secondary primary malignancy (SPM) that has recently been appreciated. While patients (pts) with MM have an 8%-17% risk of developing an SPM while on lenalidomide (R) maintenance following autologous stem cell transplant (ASCT), trALL has been rarely observed. Little is known about the characteristics of trALL in pts with MM compared to pts who had other malignancies prior to the development of trALL. We define tALL as any prior exposure to cytotoxic chemotherapy and/or radiation for another malignancy, and herein, we report a comparative analysis of characteristics and outcomes of pts with trALL with and without MM from the Mayo Clinic Cancer Center (MCCC).
Methods: We performed a systematic search in the 3-site MCCC registry and included all pts diagnosed and received at least 1 cycle of ALL-directed therapy and/or underwent allogeneic transplantation (AlloHCT) for ALL between 2007-2020. All cases of trALL in this series are B-ALL. Comparisons of characteristics between trALL patients with and without MM were made using a Wilcoxon rank sum test (continuous variables) or Fisher's exact test (categorical variables). Time-to-event variables were compared using unadjusted Cox proportional hazards regression models.
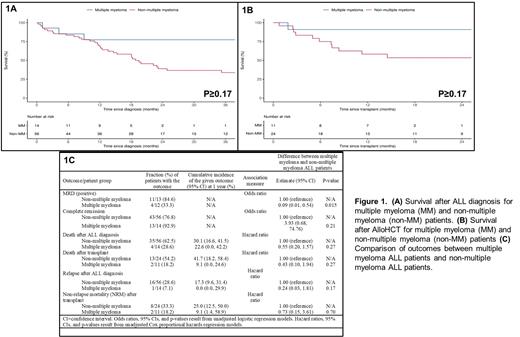
Results: 70 trALL pts (14 MM [20%], 56 non-MM [80%]) were identified during the study period. The median age at trALL diagnosis was 63.9 (range 47-71) for MM pts and 63.1 (range 18.2-84) for non-MM pts. Median follow-up from trALL diagnosis was 1.42 years (range 0.02-10.6 years). The most common prior non-MM malignancies among tALL pts were breast (28.6%), lymphoma (17.9%), myeloid (17.9%), and GU/GYN (14.3%). Amongst the 14 MM pts, 100% (n=10) had ISS-I disease, 8 (57%) had IgG K MM, 1 (n=10) had high risk cytogenetics, 8 (57.1%) received R-based induction , 4 (28.6%) received CyBorD induction, 4(28.6%) received VRD induction, 3 (21.4%) received RD induction. 10 (71.4%) pts were actively on R when they developed trALL, 8 (57.1%) pts received prior ASCT. The median time to develop trALL following ASCT for MM was 46.3 months (m), (95% CI 20.4-67.6), 12 (85.7%) pts received R maintenance with a median duration of 53m (95% CI 16.9-121). The median time to develop trALL after initiation of R maintenance was 61.2m (95% CI 16.9-123.4). The most common trALL induction regimen received was HyperCVAD which was used in 71.4% of MM pts and 51.8% of non-MM pts. In comparison to non-MM pts, MM patients had a significantly lower WBC at diagnosis (Median: 2.0 vs. 5.8, P=0.005),no t(9;22) BCR/ABL1 cytogenetics (0.0% vs. 35.7%, P=0.008), a higher frequency of hypodiploid/near-triploid (Ho-Tri) cytogenetics (42.9% vs. 12.5%, P=0.009), and a lower frequency of prior radiation therapy (7.1% vs. 44.6%, P=0.012). MM pts had a higher frequency of AlloHCT for trALL (71.4% vs. 42.9%, P=0.075) and were more commonly in complete response (CR) at transplant (100.0% vs. 70.8%, P=0.078). No trALL in MM pts were Ph-like ALL. There was a statistically significant difference in time to development of trALL between previous malignancy locations (P=0.018), with GU/GYN (Median: 9 years) having the longest time and myeloid (Median: 3 years) having the shortest time; MM was 6 years. In the overall pt cohort, 56 (80%) pts achieved a complete response after induction and 60% (n=25) were minimal residual disease (MRD)+ after induction. Pts with MM had a significantly lower likelihood of being MRD+ after induction (OR=0.09, P=0.015). Overall, 24.3% of pts relapsed after induction therapy for trALL; 39 (55.5%) died after trALL diagnosis including 43% (n=35) who died after AlloHCT. Although pts with MM tended to have better outcomes compared to non-MM trALL pts in terms of overall survival after trALL diagnosis (Figure 1A), survival after AlloHCT (Figure 1B), relapse rate after trALL diagnosis and non-relapse mortality after AlloHCT, none of these findings were statistically significant in this small cohort (Figure 1C).
Conclusion: trALL is a unique SPM that can occur in pts with MM. It is associated with Ho-Tri cytogenetics and is BCR/ABL1 negative. The majority of MM pts that developed trALL had undergone prior ASCT and were on R maintenance when they developed trALL. Despite being more likely to have adverse cytogenetics features, MM-associated trALL has similar survival compared to non-MM-associated trALL which speaks to the poor survival outcomes of this SPM. Further research on trALL in MM pts is merited.
Litzow: Amgen: Research Funding; Pluristem: Research Funding; AbbVie: Research Funding; Actinium: Research Funding; Astellas: Research Funding; Omeros: Other: Advisory Board; Jazz: Other: Advisory Board; Biosight: Other: Data monitoring committee. Bergsagel: Janssen: Consultancy. Fonseca: Scientific Advisory Board: Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; Caris Life Sciences: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy; Patent: Prognosticaton of myeloma via FISH: Patents & Royalties; Bayer: Consultancy; Takeda: Consultancy; Celgene: Consultancy; Amgen: Consultancy; BMS: Consultancy; Mayo Clinic in Arizona: Current Employment; Juno: Consultancy; Kite: Consultancy; Aduro: Consultancy; OncoTracker: Consultancy, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy; Novartis: Consultancy; Merck: Consultancy; Sanofi: Consultancy; Pharmacyclics: Consultancy; Janssen: Consultancy. Dispenzieri: Takeda: Research Funding; Alnylam: Research Funding; Pfizer: Research Funding; Oncopeptides: Consultancy; Sorrento Therapeutics: Consultancy; Janssen: Consultancy, Research Funding. Murthy: CRISPR Therapeutics: Research Funding. Ailawadhi: GSK: Consultancy, Research Funding; Sanofi: Consultancy; Pharmacyclics: Consultancy, Research Funding; Takeda: Consultancy; Medimmune: Research Funding; Xencor: Research Funding; Genentech: Consultancy; Janssen: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Cellectar: Research Funding; Beigene: Consultancy; BMS: Consultancy, Research Funding; AbbVie: Consultancy; Karyopharm: Consultancy; Ascentage: Research Funding. Foran: actinium: Research Funding; taiho: Honoraria; bms: Honoraria; servier: Honoraria; pfizer: Honoraria; novartis: Honoraria; OncLive: Honoraria; abbvie: Research Funding; boehringer ingelheim: Research Funding; takeda: Research Funding; trillium: Research Funding; aptose: Research Funding; revolution medicine: Honoraria; syros: Honoraria; sanofi aventis: Honoraria; certara: Honoraria; gamida: Honoraria; kura: Research Funding; h3bioscience: Research Funding; aprea: Research Funding; sellas: Research Funding; stemline: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal